FDA Releases Draft Guidance on Emerging Manufacturing Technology
January 10, 2016 - BioPharm Intl.
By Caroline Hroncich

Combining Microbioreactors and Advanced Statistical Techniques to
December 17, 2015 - BioPharm Intl.
Use of a subspace model is a viable method to characterize process space variables and optimize process performance.
By Colin Jaques
…
Biacore Concentration and Ligand-Binding Analyses
December 17, 2015 - Cytiva
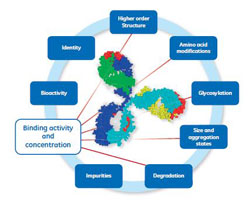 …
…
Fed-batch supplements to boost cell culture performance
December 17, 2015 - Cytiva
 To increase the yield of a target protein, fed-batch cult
To increase the yield of a target protein, fed-batch cult
Implications of Cell Culture Conditions on Protein Glycosylation
December 3, 2015 - BioPharm Intl.
The authors present a review of the techniques commonly used for glycosylation analysis.
By Michiel E. Ultee, PhD, Dr. Richard Easton
…
Educational Protein Purification Videos
December 3, 2015 - Cytiva
You can shape tomorrow’s researchers today by providing them with skills essential for modern protein research laboratories. Here you will find useful resourc
…
GMP Challenges for Advanced Therapy Medicinal Products
December 3, 2015 - Pharmaceutical Technology
Finalizing GMP requirements and quality standards for the development, manufacture, and clinical testing of ATMPs in the EU is proving to be a complex t
…
Antibody Purification Handbook
December 3, 2015 - Cytiva
 The diversity of the antibody-antigen interaction
The diversity of the antibody-antigen interaction
CMOs Continue to Improve Overall Biomanufacturing Performance
November 19, 2015 - BioPharm Intl.
Better process development is creating industry benchmarks for bioprocessing.
By Eric Langer
…
Selecting the Right Viral Clearance Technology
November 19, 2015 - BioPharm Intl.
By Cynthia Challener, PhD
