Developing Cleaning-in-Place Protocols
March 16, 2016 - Cytiva
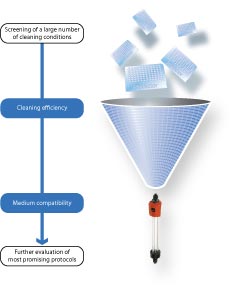 Cleaning conditions for chromatography media can be screened in a
Cleaning conditions for chromatography media can be screened in a
Antibody Production in Microbial Hosts
February 18, 2016 - BioPharm Intl.
The authors review the status of expression of antibodies in microbial hosts and present the recent advances in the production of aglycosylated antibodi
…
Innovative Therapies Require Modern Manufacturing Systems
February 18, 2016 - BioPharm Intl.
FDA and industry see progress and challenges in bringing cutting-edge medicines to patients.
By Jill Wechsler
A Q&A With Jeff Carter on Trends in Single-Use Technologies
February 18, 2016 - Process Development Forum
Process Development Forum speaks with Jeff Carter, Strategic Projects Leader at Cytiva, and first Vice Chair on the executive board of the Bio-
…
Framing Biopharma Success in 2016
February 4, 2016 - BioPharm Intl.
Corporate restructurings, regulatory initiatives, and biosimilars will shape biopharma development in 2016.
By Rita C. Peters
…
KUBio modular biopharm facility built in Germany, assembled in Ch
January 22, 2016 - Cytiva
 Cytiva is supplying a full biopharmaceutical processing so
Cytiva is supplying a full biopharmaceutical processing so
Tools for Continuous Bioprocessing Development
January 21, 2016 - BioPharm Intl.
Could perfusion microbioreactors bring more agility to biomanufacturing?
By Rajeev J. Ram
In semiconductor manufact
…
Going Small to Achieve Success on the Commercial Scale
January 21, 2016 - BioPharm Intl.
Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.
By Cynthia A. Cha
…
Biopharma in 2015: A Year for Approvals and Innovations
January 14, 2016 - Process Development Forum
 Insiders
Insiders
mAbs to Watch in 2016
January 10, 2016 - BioPharm Intl.
By Randi Hernandez
Of the 45 new molecular entities and new therapeutic biological products approved by FDA in 2015, nine products were monoclonal an
…