Real Time Continuous Microbiological Monitoring
October 10, 2016 - BioPharm Intl.
Laser-induced fluorescence, a rapid microbiology method for real-time airborne particle and microbial monitoring, enhances sterility assurance in pharma
…
Establishing Acceptance Criteria for Analytical Methods
October 10, 2016 - BioPharm Intl.
Knowing how method performance impacts out-of-specification rates may improve quality risk management and product knowledge.
By Thom
…
Mixing dynamics in a large volume single use mixer
September 29, 2016 - Cytiva
 Large volume single-use technology mixers can be used f
Large volume single-use technology mixers can be used f
High-Throughput Process Development Handbook
September 29, 2016 - Cytiva
Time-to-clinic and time-to-market are two key factors for successful biopharmaceutical development. Efficient development of the manufacturing process i
Modeling Bioreactor Performance
September 29, 2016 - BioPharm Intl.
Model effectiveness is determined by the quality and composition of the data inputs.
By Cynthia A. Challener
Efforts Accelerate to Streamline Postapproval Change Process
September 29, 2016 - Pharm Tech
Manufacturers and regulatory authorities seek coordinated lifecycle management policies.
By Jill Wechsler
Overcoming Buffer Challenges With In-Line Conditioning
September 14, 2016 - Cytiva
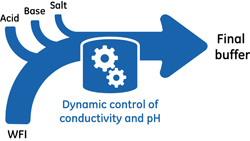 Preparation and storage of the large number of buffers requi
Preparation and storage of the large number of buffers requi
Process Chromatography Selection for Downstream Processing Applic
September 14, 2016 - BioPharm International
Industry experts discuss best practices for selecting a separation technology.
By Caroline Hroncich
Safety Drives Innovation in Animal-Component-Free Cell-Culture Me
September 14, 2016 - BioPharm Intl.
Advances in cell culture media technology have helped achieve safer biologics.
By Tom Fletcher, Holden Harris
Innovation vs. Capacity: How CMOs Compete
September 14, 2016 - BioPharm Intl.
The strategies of an innovation-driven CMO may be different than a capacity-driven CMO.
By Jim Miller

