Automating Processes in Upstream Processing
December 8, 2016 - BioPharm Intl.
By Susan Haigney
BioPharm International spoke with Trevor Marshall, director of enterprise systems integration at
…
Efficient Purification of Pneumococcal Polysaccharides in a Chrom
November 28, 2016 - Cytiva
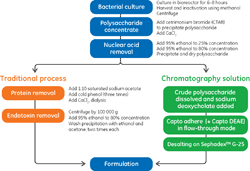 This application note demonstrates the purificatio
This application note demonstrates the purificatio
Efficient Purification of the Pertussis Antigens Toxin, Filamento
November 28, 2016 - Cytiva
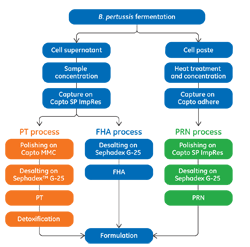 This application note describes the purification of pertussis tox
This application note describes the purification of pertussis tox
Advancing Single-Use Technology Through Collaboration
November 11, 2016 - BioPharm Intl.
By working together to harmonize the highly variable steps within the biopharmaceutical manufacturing process, both end users and suppliers are making s
…
Validation of the production of influenza virus in ReadyToProcess
November 10, 2016 - Cytiva
 This application note describes the validation of the single-u
This application note describes the validation of the single-u
A Platform Approach to Purification of Antibody Fragments
November 10, 2016 - Cytiva
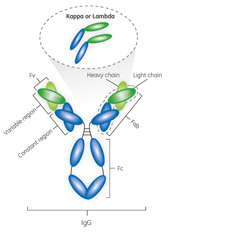 Antibody fragments constitute a promising class of biopharmac
Antibody fragments constitute a promising class of biopharmac
Development of column packing methods based on pressure flow meas
November 10, 2016 - Cytiva
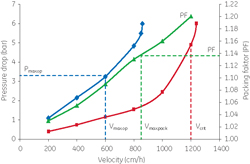 Scale-up of a chromatography process might appear
Scale-up of a chromatography process might appear
Walk Away and Do More Screening Preps
November 10, 2016 - Cytiva
Developing a process or looking for a clone of interest often requires screening large numbers of samples. Many analyses require purified protein, and substanti
…
Improving Process-Scale Chromatography
November 10, 2016 - BioPharm Intl.
Advances in technology are increasing the productivity and efficiency of commercial-scale chromatography bioprocesses.
By Cynthia Ch
…
Defining Risk Assessment of Aseptic Processes
October 26, 2016 - PharmTech
Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the assessment of risk in the processing of intravenous injectable d
…