Identify the Error Control Plan
Make sure to measure and record uncontrolled factors during the study. Analyst name, equipment ID, out time, hold times, ambient temperature, temperature at the beginning and end of an operation, transfer times, pH, and incubation time may hold valuable information on factors that impact the method. What factors will be restricted or held constant during the study? Do you need to block for batch, lot, sample prep, or instruments that may have an influence on the reportable result?
Analyze the DOE and Determine the Settings and Design Space
Use a good multiple regression/analysis of covariance (ANCOVA) software package that allows the DOE factors and any uncontrolled variables to be correctly evaluated. Analyze the study and determine settings and processing conditions that improve method precision and minimize bias errors (see Figure 5). When using statistics from the method (e.g., CV, mean, standard deviation), rather than raw data, make sure to weigh the analysis by the number of replicates or duplicates to assure statistical tests and confidence intervals are meaningful. Determine the design space and allowable ranges for all key factors that influence the method.
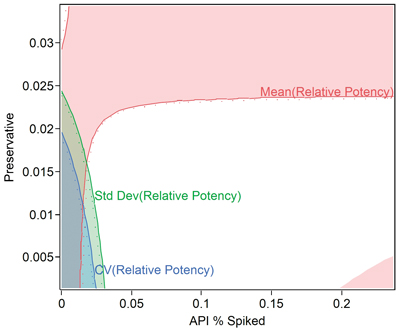
Verify the Model and Determine the Impact of the Method on Specifications and Capability
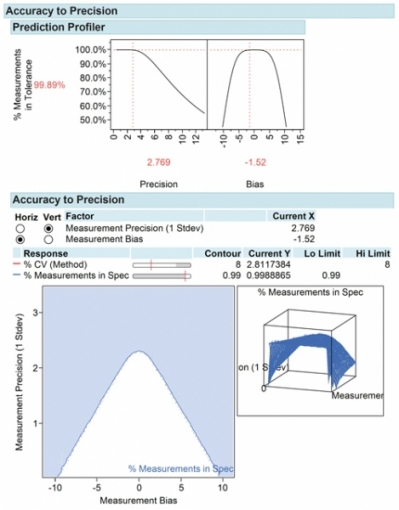
Run confirmation tests to confirm settings improve precision, linearity, and bias. Evaluate the impact of the method on product acceptance rates and process capability. Using an accuracy-to-precision (ATP) model (4), it is possible to visualize the relationship of precision and accuracy on product acceptance rates. The ATP model shows how changes in precision and accuracy impact product acceptance rates and the assay error design space relative to product acceptance specification limits.
The attention paid to method development, validation, and control will greatly improve the quality of drug development, patient safety, and predictable, consistent outcomes (see Figure 6).
Summary
Design of experiment is a powerful and underutilized development tool for method characterization and method validation. Analytical professionals need to be comfortable using it to characterize and optimize the analytical method. If used properly and during development, DOE will provide significant improvements in precision and a reduction in bias errors. It will further help to avoid costly and time consuming validation studies as concentrations are modified in formulations and dosing schemes are changed for drug product and drug substance.
References
1. ICH, Q2(R1) Validation of Analytical Procedures: Text and Methodology (ICH, 2005).
2. ICH, Q8(R2) Pharmaceutical Development (ICH, 2009)
3. ICH, Q9 Quality Risk Management (ICH, 2006).
4. T.A. Little, Assay Development and Method Validation (2014).
About the Author
Thomas A. Little is president of Thomas A. Little Consulting, [email protected].