Microbiological Testing: Time is of the Essence
August 13, 2016 - BioPharm Intl.
Pressures to accelerate current and next-gen therapies are challenging traditional microbiological testing methods.
By Cynthia A. Cha
…
Single-use technologies in downstream process intensification
August 13, 2016 - Cytiva
 In bioprocessing, thorough clean
In bioprocessing, thorough clean
Vocabulary of the chromatographer
August 13, 2016 - Cytiva
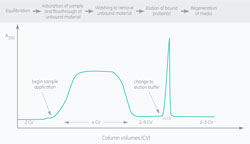 Similar to other technical areas, chromat
Similar to other technical areas, chromat
Managing Biomanufacturing Capacity Expectations
July 13, 2016 - BioPharm Intl.
Capacity for complex therapeutics is becoming increasingly difficult to predict.
By Randi Hernandez
Maximize resin usage with continuous bioprocessing
July 13, 2016 - Cytiva
Continuous processing has proved a very successful model in many industries. As such, there has been a growing interest in using continuous concepts also in bio
…
Affinity chromatography Handbook - Vol. 3: Specific Groups of Bio
July 13, 2016 - Cytiva
Affinity chromatography (AC) separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand coupled
…
Biosimilars: Making the Switch Comes with Challenges
June 29, 2016 - BioPharm Intl.
More efforts are needed to raise awareness of biosimilars among physicians and patients in Europe and address scepticisms about the quality and safety o
…
ReadyToProcess™ Prepacked Columns
June 29, 2016 - Cytiva
 ReadyToProcess columns are high-performance bioproces
ReadyToProcess columns are high-performance bioproces
Affinity chromatography Handbook: Vol. 2 Tagged Proteins
June 29, 2016 - Cytiva
_250.jpg) This handbook is intended for those interested in
This handbook is intended for those interested in
Make buffer preparation more time and cost efficient with inline
June 29, 2016 - Cytiva
Simplify Buffer Preparation
The volume and number of buffers for a typical downstream process can be considerable.