A deep dive into optimizing AAV capture and polishing to maximize
December 15, 2023 - Cytiva

As demand for AAV vectors grows, so does the need for scalable an
…
Video: Standardize recombinant protein purification process devel
December 15, 2023 - Cytiva

Cytivaâ„¢ Protein Selectâ„¢ resin is an affinity chromatography r
…
Report from the 6th International HTPD Conference
December 1, 2023 - Cytiva
The biggest challenge is still the people and Quantitative Structure-Activity Relationship (QSAR) plus mechanistic modeling were two key take-home messages f
…
Insights from process developers - top challenges in recombinant
December 1, 2023 - Cytiva
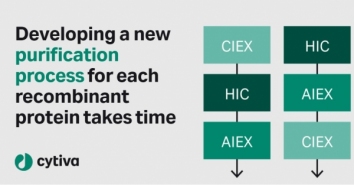
You might be one of the many process de
Getting your investigational drug regulatory ready
December 1, 2023 - Cytiva

Navigating regulatory requirements can be an o
…
Democratizing GMP Manufacturing for the New Therapeutic Pipeline
December 1, 2023 - BioPharm International
PharmTech Europe
discusses technology that enable
Developing oligonucleotide therapeutics with confidence
November 17, 2023 - Cytiva
In this video, we will look at
Good modeling practice: workflow and case study
November 17, 2023 - Cytiva

Learn more about a recommended mechanisti
…
Top challenges in recombinant protein purification process develo
November 17, 2023 - Cytiva, BioPharm International
…
Discussing the Origins of the mRNA Therapeutics Field
November 17, 2023 - BioPharm International

Andy Geall, co-founder and chief development officer at Replicate
…