2019’s Top Bioprocessing Trends and What to Expect in 2020
January 16, 2020 - Bioprocess Development Forum
 2019 proved to be an exciting year for biopharmaceutical developers a
2019 proved to be an exciting year for biopharmaceutical developers a
Risk Assessment: Studying Chromatography Resin Variability
December 16, 2019 - Cytiva
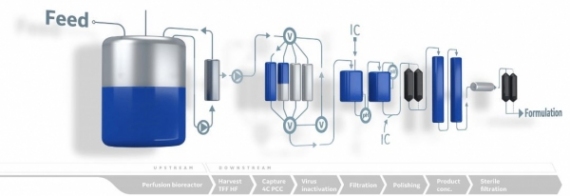
A Continuous Confidence Boost
December 16, 2019 - Cytiva
 …
…
The Evolving Role of Starting Materials in Cell and Gene Therapy
December 16, 2019 - BioPharm International
Th
…
Factors Which Impact mAb Process Scale-Up
December 16, 2019 - Cytiva
<iframe width="560" height="315" src="https://www.youtube.com/embed/8fSXqqapyx0" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-medi
…
Raw Material Variability: The Need for Deeper Process Understandi
December 5, 2019 - Cytiva
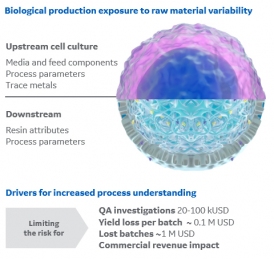 Principles and methodologies for biopharmaceutical
Principles and methodologies for biopharmaceutical
Process Development and Optimization Using the ReadyToProcess WAV
December 5, 2019 - Cytiva
Download this application note to learn about the technical feasibility of the Method editor and operations in dual mode (where two parallel cultures are contro
…
New Therapies Present Scaling Challenges
December 5, 2019 - BioPharm International
Ne
…
A Diverse Landscape of Patent Issues Seen in the US, UK, and the
December 5, 2019 - BioPharm International
A
…
Cytiva to Expand KUBio Through Close Collaboration with Pharmadul
December 5, 2019 - Cytiva
 Cytiva has announced it will be working closely with Pharmadul
Cytiva has announced it will be working closely with Pharmadul