Improving mAb Manufacturing Productivity by Optimizing Buffer and
July 30, 2020 - BioPharm International
Understanding and Controlling Raw Material Variation in Cell Cult
July 16, 2020 - Cytiva
 …
…
Controlling Cell Culture Process Variability Through Supplier-Ena
July 16, 2020 - Cytiva

Eliminating Residual Impurities Starts with a Strategic Plan
July 16, 2020 - BioPharm International
El
…
Next Generation Process Chromatography
July 16, 2020 - Cytiva
 Chromatography is an essential part in the production of al
Chromatography is an essential part in the production of al
Step Up to GMP Manufacturing or Outsource to a CDMO: Consideratio
July 2, 2020 - Cytiva

Cytiva Upgrades Massachusetts Contract Biomanufacturing Site with
July 2, 2020 - Cytiva

Good Manufacturing Practices: Challenges with Compliance
July 2, 2020 - BioPharm International
Case Study: High Throughput mAb Purification Using Fibro
June 18, 2020 - Cytiva
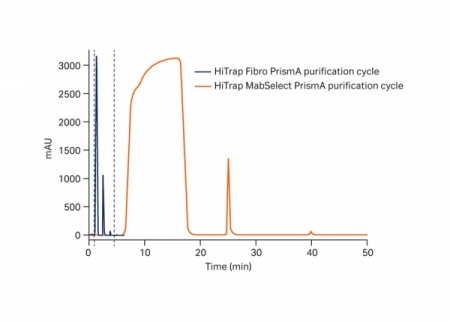 …
…
Improving Lentiviral Vector Downstream Processing Workflows
June 18, 2020 - Cytiva
Lentiviral vectors (LVV) are a common vehicle to deliver genetic material in chimeric antigen receptor (CAR) T-cell therapy and gene therapy applications. Produ
…